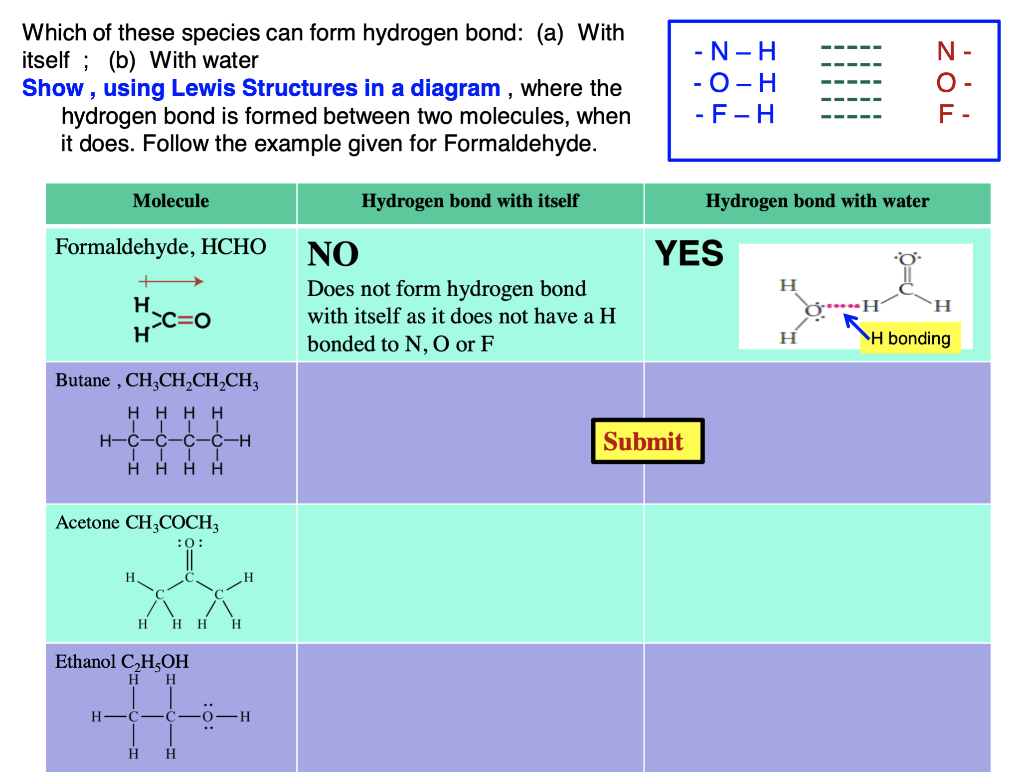
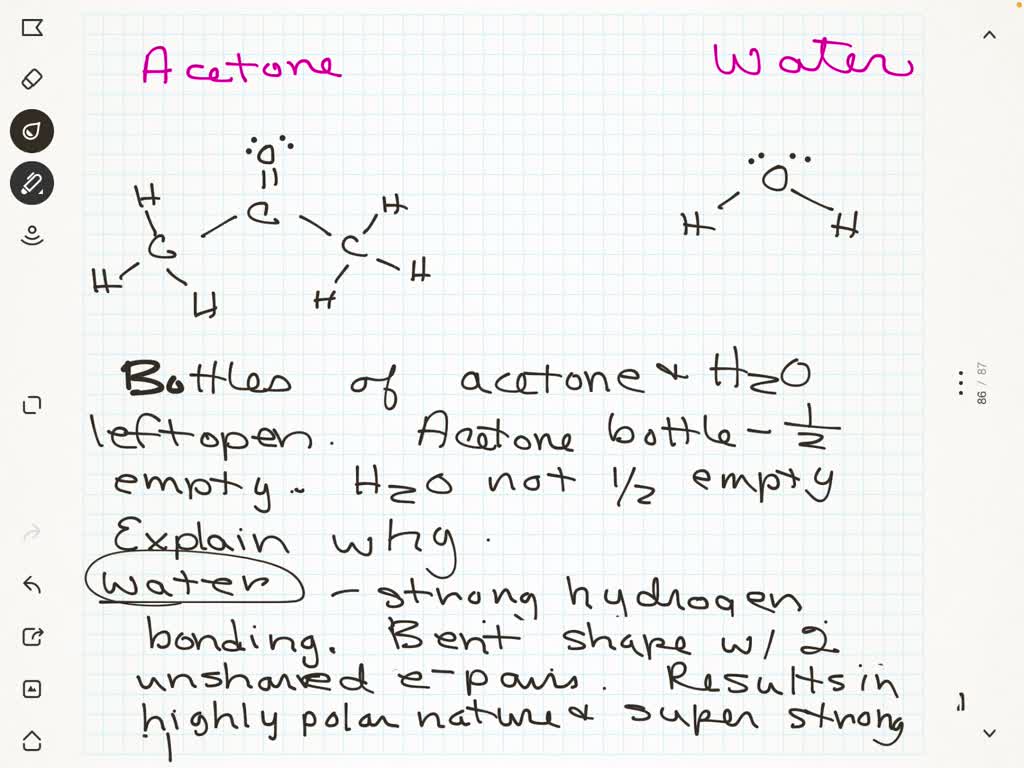
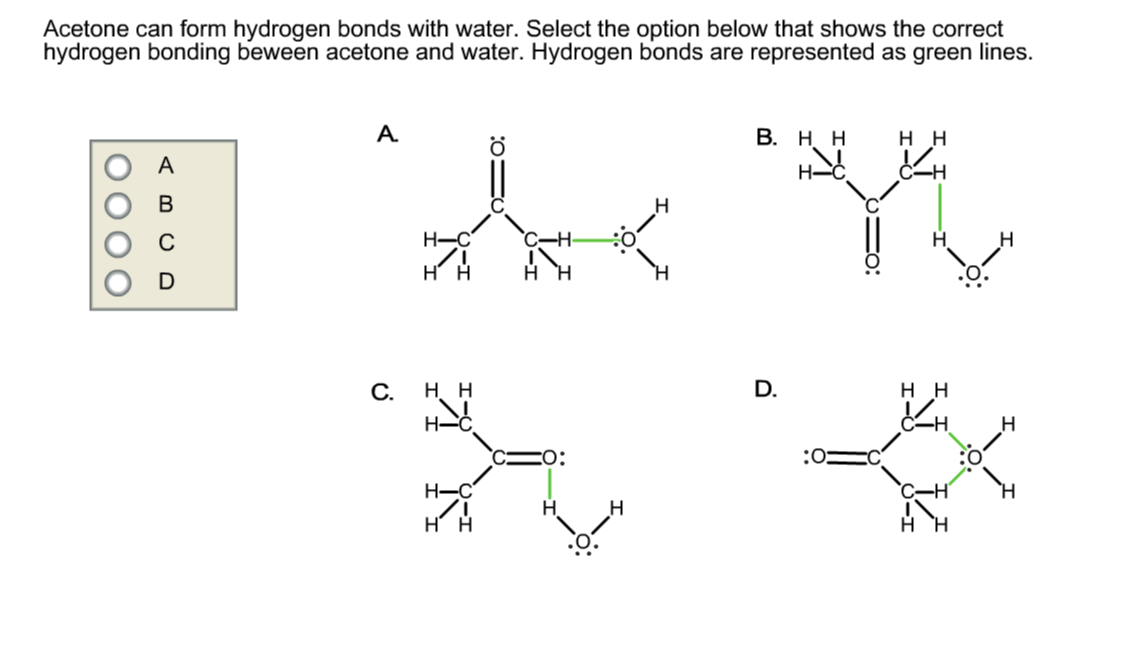
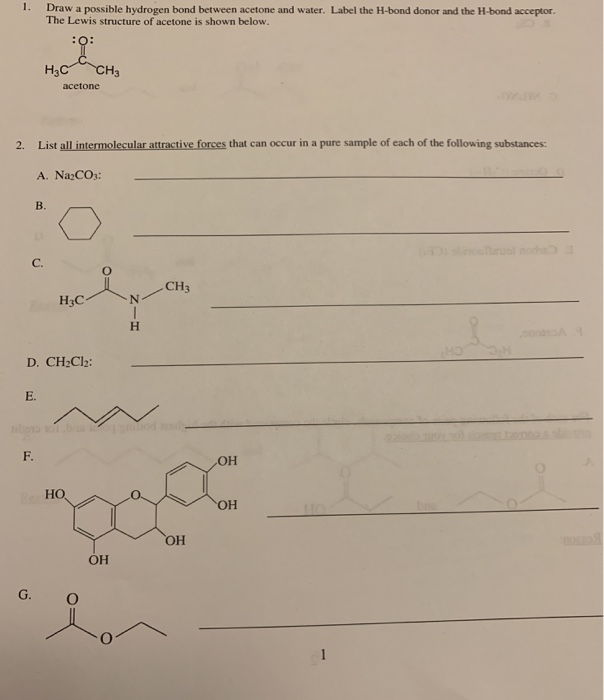
Can Acetone Form Hydrogen Bonds
Can Acetone Form Hydrogen Bonds - I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Acetone can form hydrogen bonds with water. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water.
Acetone can form hydrogen bonds with water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The water molecule forms two hydrogen bonds with acetone: Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming.
The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming.
Answered Can Butane, Acetone, and Ethanol form a… bartleby
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the.
SOLVED How many hydrogen bonds can form between an acetone molecule
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone can form hydrogen bonds with water. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of.
hydrogen bond acetone ethanol YouTube
The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water.
Acetone Lewis Structure With Polarity
The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone..
SOLVED2. Can a molecule of acetone form a hydrogen bond with water
Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming.
SOLVED SOURCE CHAPTER PROBLEM TOPIC INTRAMOLECULAR FORCES The
Four images are given which show an acetone molecule interacting with a water. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The hydrogen atom of.
Solved Acetone can form hydrogen bonds with water. Select
Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been.
Solved 1. Draw a possible hydrogen bond between acetone and
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is.
I had to draw Hbonds between water and Acetone. Did I do it correctly
I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone. Four images are given which show an acetone molecule interacting with a water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. The water molecule forms two hydrogen bonds with acetone: Acetone can.
SOLVEDWhy can't two molecules of acetone form a hydrogen bond with
The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water. Acetone does not have a hydrogen atom bonded to a highly electronegative atom, which is the primary requirement for forming. Four images are given which show an acetone molecule interacting with a water. The hydrogen atom of h 2 o is coordinated to the.
Four Images Are Given Which Show An Acetone Molecule Interacting With A Water.
The water molecule forms two hydrogen bonds with acetone: Acetone can form hydrogen bonds with water. The hydrogen atom of h 2 o is coordinated to the carbonyl group of acetone,. I haven't been able to find a reference confirming that fluoroform forms hydrogen bonds with acetone.