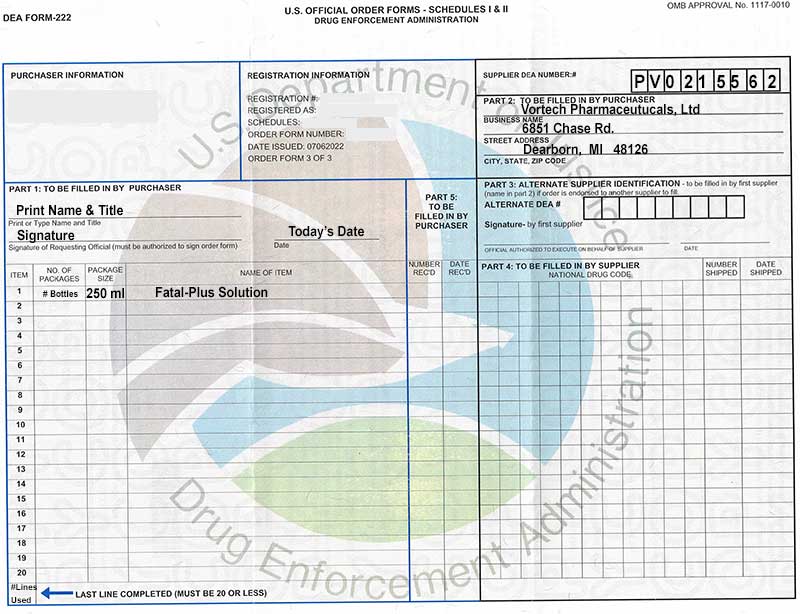
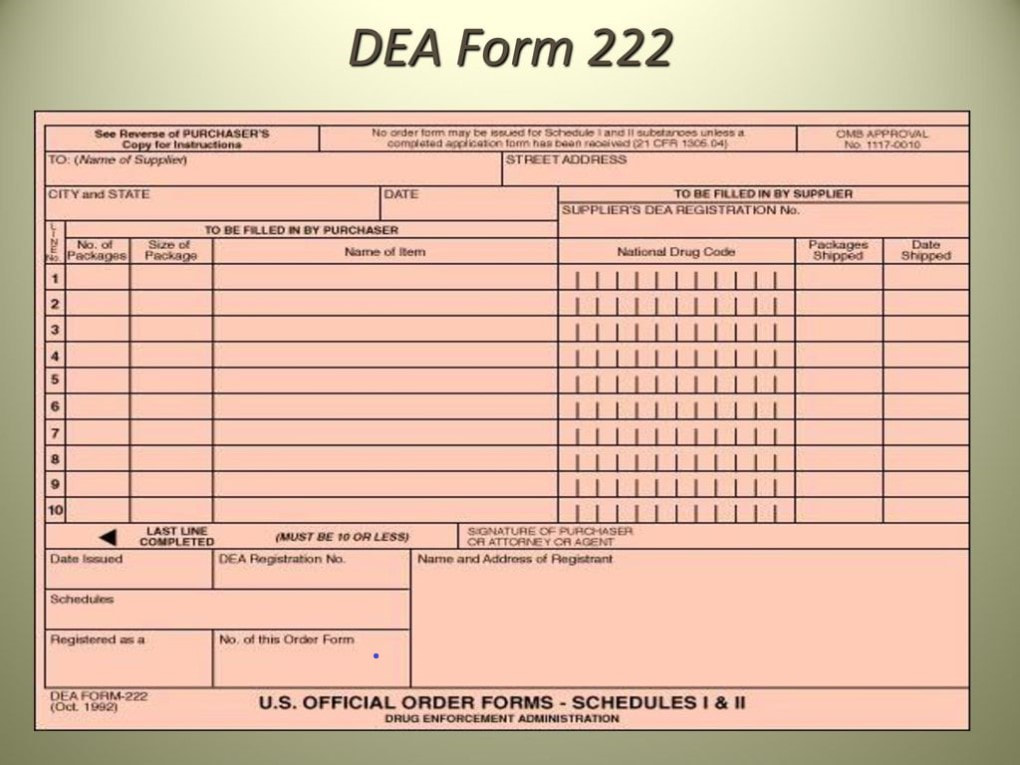
Dea 222 Form
Dea 222 Form - As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Modify dea registration to stop being a collector; Neither the controlled substances act nor its implementing regulations. The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume of order forms (dea form 222) with the pin feed tracking. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients);
Modify dea registration to stop being a collector; Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume of order forms (dea form 222) with the pin feed tracking. Neither the controlled substances act nor its implementing regulations. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance.
Neither the controlled substances act nor its implementing regulations. The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume of order forms (dea form 222) with the pin feed tracking. Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. Modify dea registration to stop being a collector;
Fillable Online DEA 222 Form Single Sheet Instructions mmscms
Modify dea registration to stop being a collector; Neither the controlled substances act nor its implementing regulations. As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate.
Printable New Dea 222 Form Printable Forms Free Online
The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume of order forms (dea form 222) with the pin feed tracking. Under 21 cfr.
How To Order Vortech Pharmaceuticals, Ltd.
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume.
Filling out the new DEA 222 form for Pharmacy returns (as the Supplier
The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large volume of order forms (dea form 222) with the pin feed tracking. As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no.
Dea 222 Form Example Complete with ease airSlate SignNow
Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Modify dea registration to stop being a collector; The drug enforcement administration (dea), office of diversion control, will accept requests from distributors that.
Printable New Dea 222 Form Printable Forms Free Online
Neither the controlled substances act nor its implementing regulations. As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution.
Dea Chapter 35 Application
Neither the controlled substances act nor its implementing regulations. Modify dea registration to stop being a collector; Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of.
DEA form 222 A Guide to the Rules and Usage
Modify dea registration to stop being a collector; Neither the controlled substances act nor its implementing regulations. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); The drug.
Medication Ordering
Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. Modify eligible dea registration to collect pharmaceutical controlled substances from.
MPS Example DEA 222 Form
The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance. As of october 30, 2021, the drug enforcement administration is implementing the mandatory use of a single sheet dea 222 order form and the triplicate dea order forms will no longer be. Under.
The Drug Enforcement Administration (Dea), Office Of Diversion Control, Will Accept Requests From Distributors That Require A Large Volume Of Order Forms (Dea Form 222) With The Pin Feed Tracking.
Modify dea registration to stop being a collector; Modify eligible dea registration to collect pharmaceutical controlled substances from ultimate users (e.g., patients); Under 21 cfr 1305.03, the completion of dea form 222 is required for each distribution of a schedule i or ii controlled substance. Neither the controlled substances act nor its implementing regulations.
As Of October 30, 2021, The Drug Enforcement Administration Is Implementing The Mandatory Use Of A Single Sheet Dea 222 Order Form And The Triplicate Dea Order Forms Will No Longer Be.
The dea regulations provide that either a dea form 222 or its electronic equivalent is required for each distribution of a schedule i or ii controlled substance.