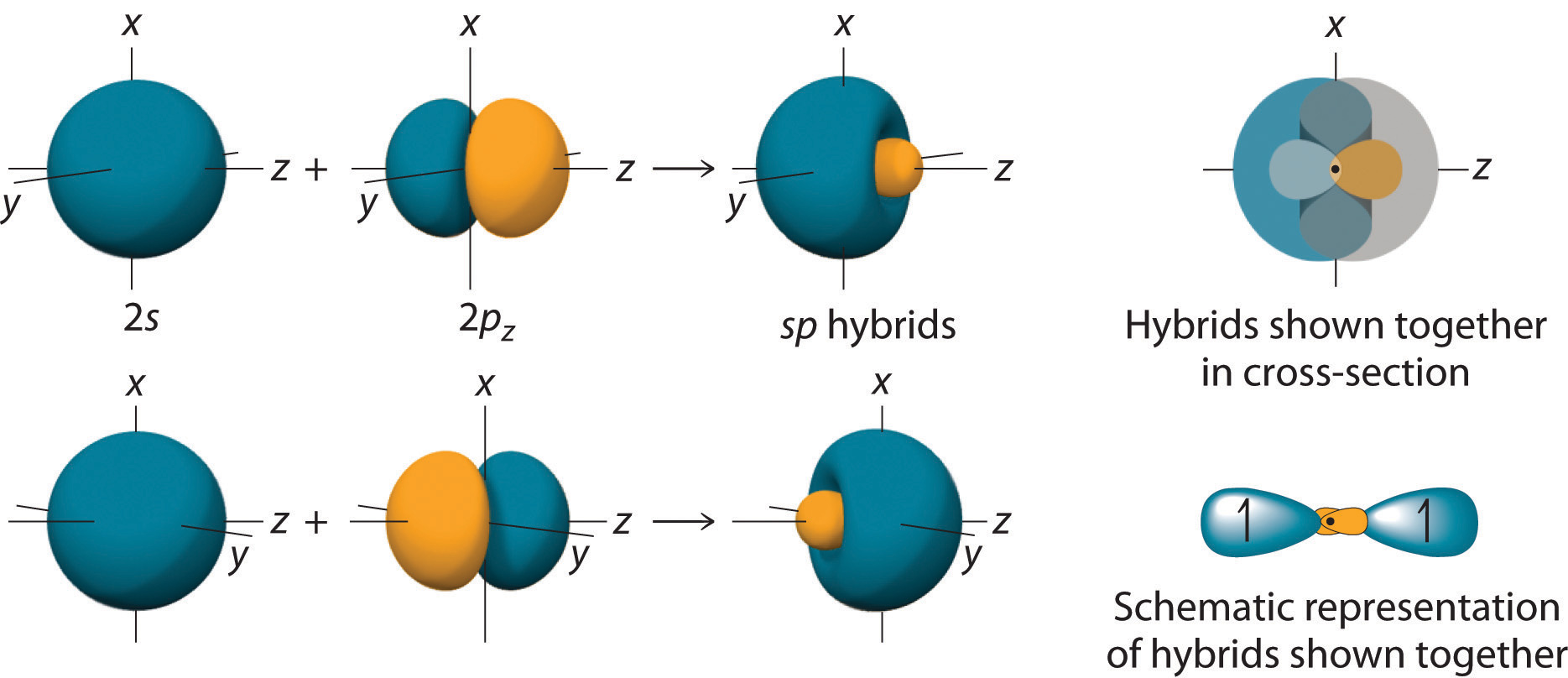
Hybrid Orbitals Overlap To Form
Hybrid Orbitals Overlap To Form - The type of hybrid orbitals formed in a bonded atom depends on. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the. Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. The type of hybrid orbitals formed in a bonded atom depends on. In the following sections, we shall discuss the. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
Identify The Set Of Hybrid Orbitals Shown Below.? New Update
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. The type of hybrid orbitals formed in a bonded atom depends on. In the following sections, we shall discuss the. Hybrid orbitals overlap to form σ bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
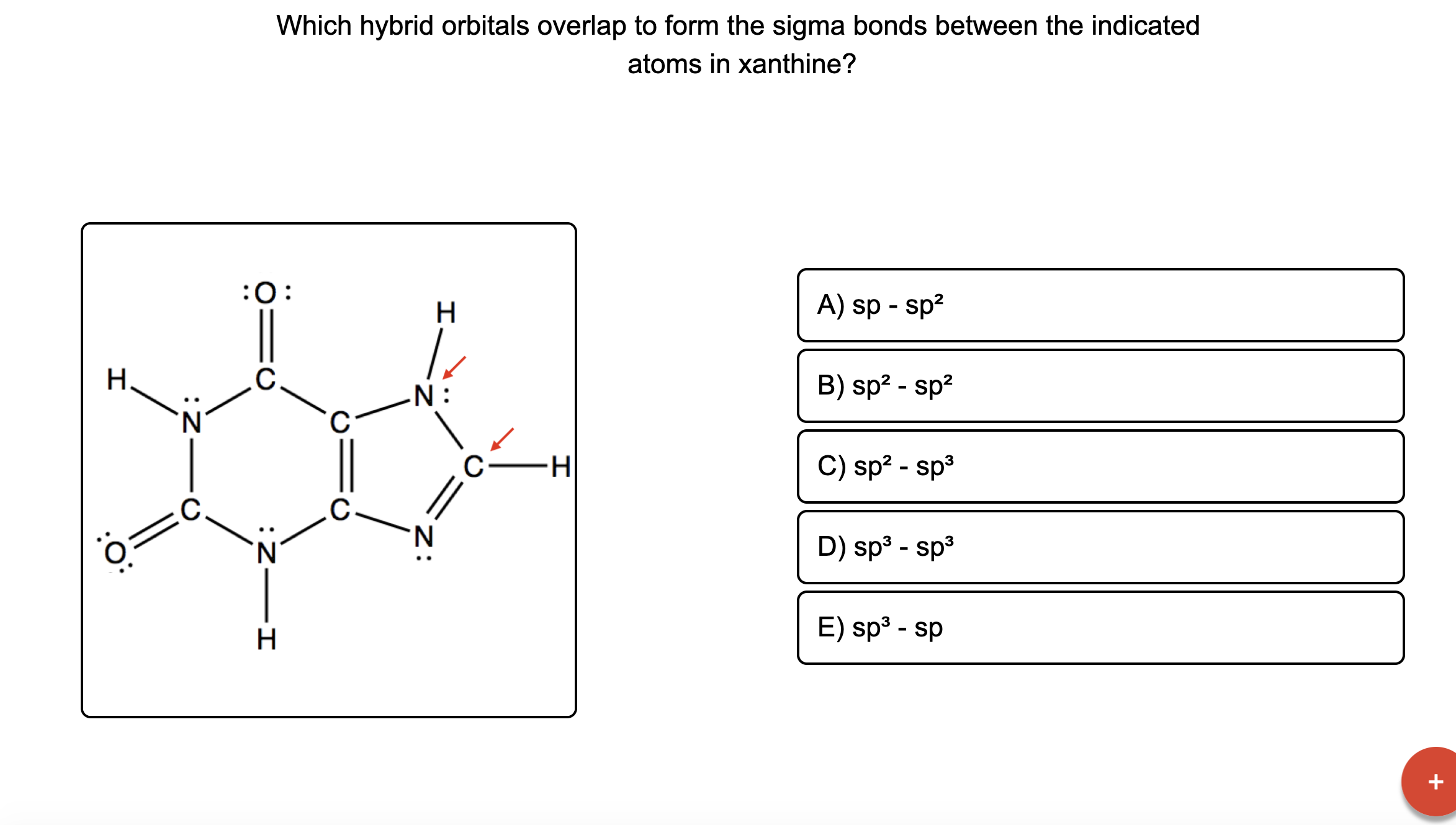
[ANSWERED] H o Z C 0 O H Which hybrid orbitals overlap to form the Kunduz
Hybrid orbitals overlap to form σ bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the. The type of hybrid orbitals formed in a bonded atom depends on. Unhybridized orbitals overlap to form π bonds.
hybrid orbitals infographic. Linus Pauling's explanation of bonding
The type of hybrid orbitals formed in a bonded atom depends on. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. Unhybridized orbitals overlap to form π bonds. Hybrid orbitals overlap to form σ bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
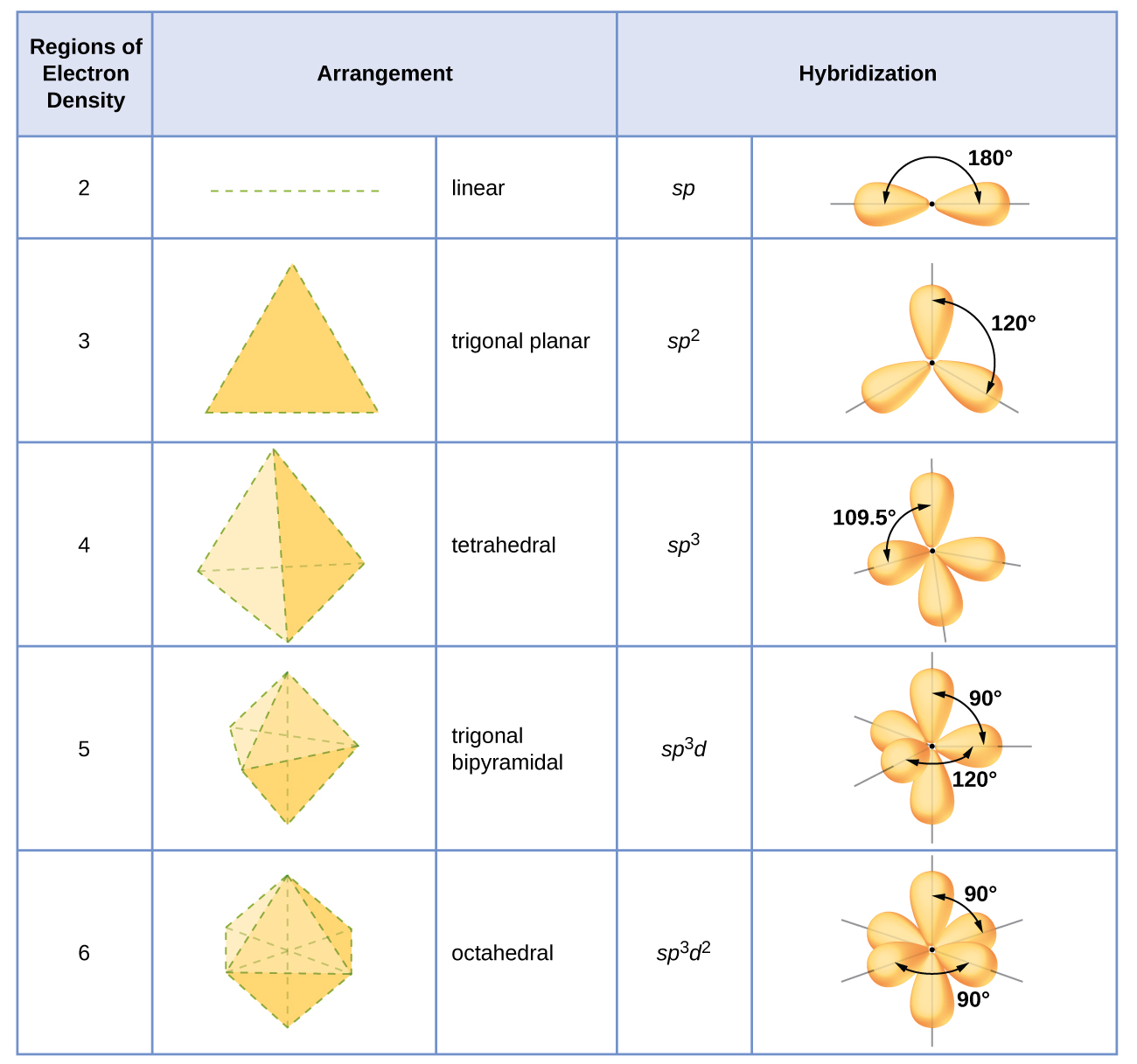
9.5 Hybrid Orbitals Chemistry LibreTexts
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the. Unhybridized orbitals overlap to form π bonds. The type of hybrid orbitals formed in a bonded atom depends on. Hybrid orbitals overlap to form σ bonds.
Answered Which hybrid orbitals overlap to form… bartleby
In the following sections, we shall discuss the. Unhybridized orbitals overlap to form π bonds. Hybrid orbitals overlap to form σ bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. The type of hybrid orbitals formed in a bonded atom depends on.
Hybrid orbitals overlap with ligand orbitals that can donate electron pai..
Hybrid orbitals overlap to form σ bonds. The type of hybrid orbitals formed in a bonded atom depends on. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the.
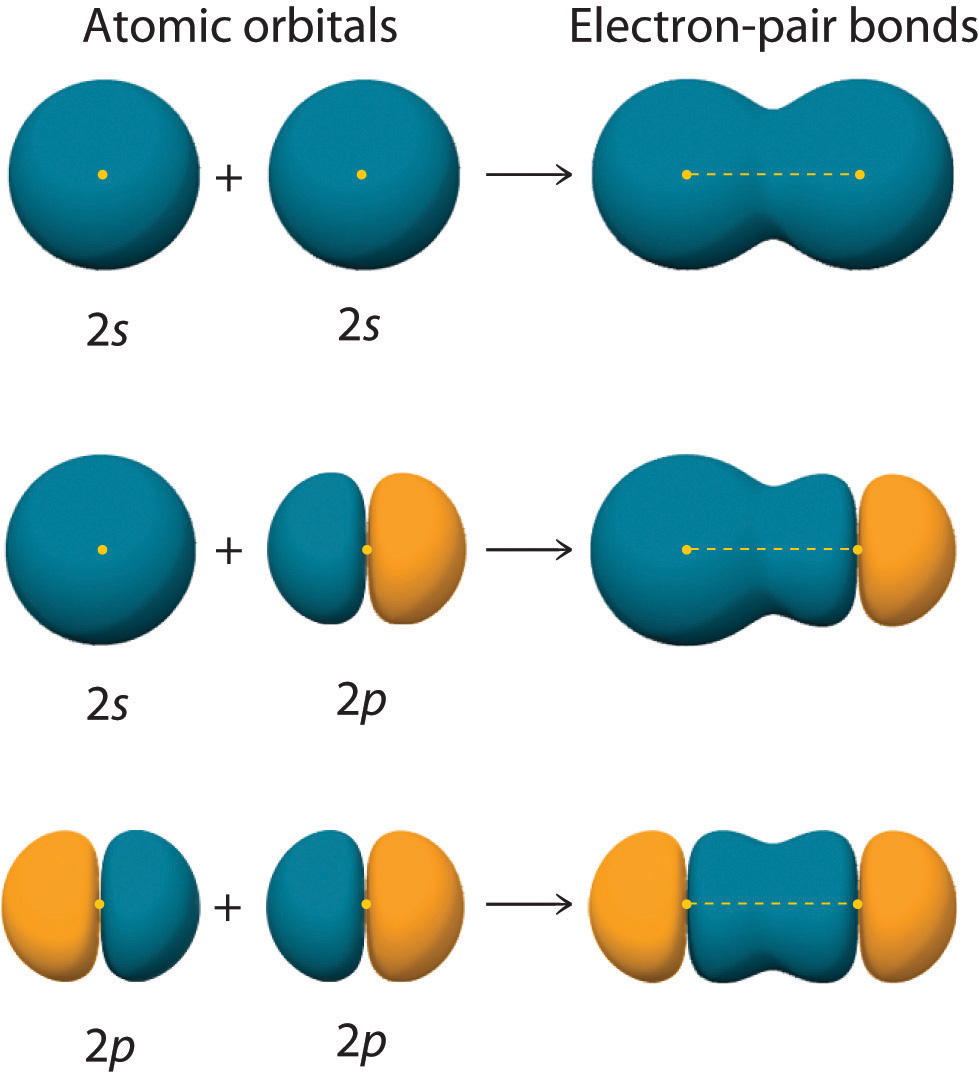
2p Orbitals
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Unhybridized orbitals overlap to form π bonds. The type of hybrid orbitals formed in a bonded atom depends on. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. In the following sections, we shall discuss the.
8.2 Hybrid Atomic Orbitals Chemistry LibreTexts
All orbitals in a set of hybrid orbitals are equivalent in shape and energy. Unhybridized orbitals overlap to form π bonds. The type of hybrid orbitals formed in a bonded atom depends on. Hybrid orbitals overlap to form σ bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when.
Which hybrid orbitals overlap to form the sigma bonds between the
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. The type of hybrid orbitals formed in a bonded atom depends on. In the following sections, we shall discuss the. Hybrid orbitals overlap to form σ bonds. All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
How To Draw Hybridization Orbitals
The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. The type of hybrid orbitals formed in a bonded atom depends on. Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds. In the following sections, we shall discuss the.
The Type Of Hybrid Orbitals Formed In A Bonded Atom Depends On.
Hybrid orbitals overlap to form σ bonds. The hybridization of orbitals is favored because hybridized orbitals are more directional which leads to greater overlap when. All orbitals in a set of hybrid orbitals are equivalent in shape and energy. In the following sections, we shall discuss the.

![[ANSWERED] H o Z C 0 O H Which hybrid orbitals overlap to form the Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210518000608701743-3314603.jpg?h=512)