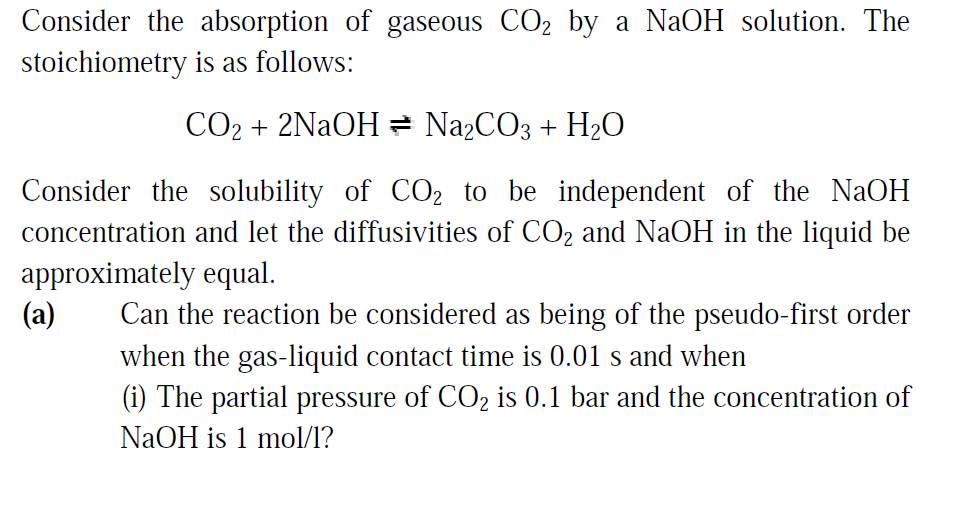Naoh Co2
Naoh Co2 - Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one.
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Sodium bicarbonate = sodium hydroxide + carbon dioxide. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Balancing of the reaction by hit and trial.
Balancing of the reaction by hit and trial. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2.
How to Write the Net Ionic Equation for NaOH + CO2 = Na2CO3 + H2O YouTube
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Sodium bicarbonate = sodium hydroxide + carbon dioxide. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Balancing of the reaction by hit and trial. Naoh +.
Figure 1 from Changes in CO2 Absorption Efficiency of NaOH Solution
Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Balancing of the reaction by hit and trial. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3.
CO2與NaOH溶液反應後產物判斷問題解析 每日頭條
Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. 1 naoh + 1.
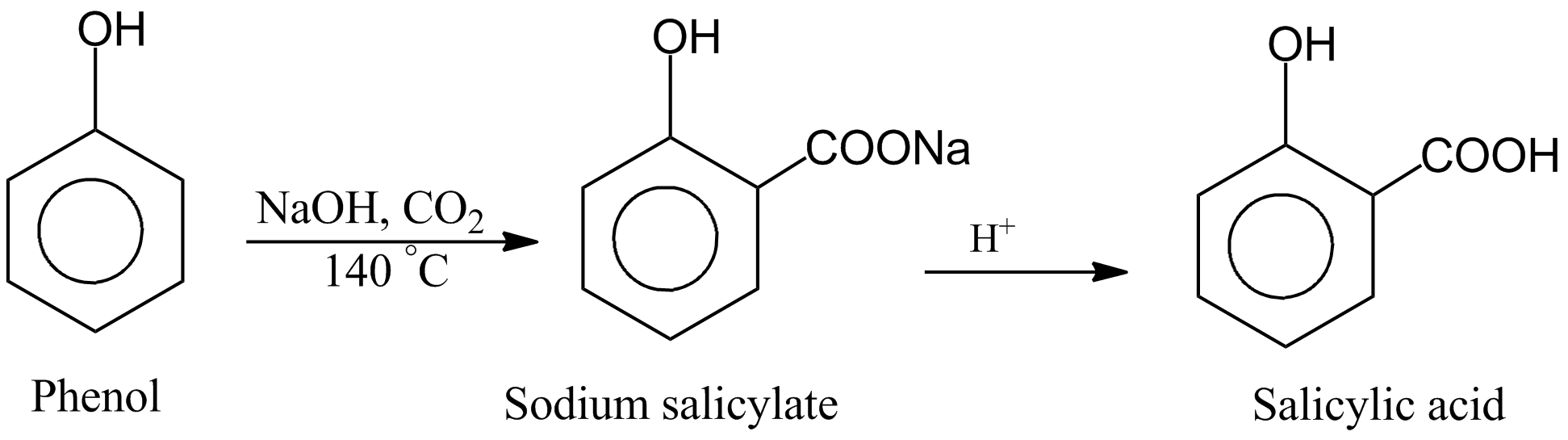
C6H6O + CO2 + NaOH 57ecrass
1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Balancing of the reaction by hit and trial. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. (a) when the alkali.
Question Video Deducing the Balanced Chemical Equation for the
Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Nahco3 = naoh + co2 is a decomposition reaction where one mole of. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react.
Consider the absorption of gaseous C02 by a NaOH
Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. (a) when the alkali ($\ce{naoh}$) solution is very dilute.
NaOH+CO2=Na2CO3+H2O. balance the chemical equation mydocumentary838
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Balancing of the reaction by hit and trial. 1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both.
NaHCO3 = NaOH + CO2
1 naoh + 1 co 2 = 1 na 2 co 3 + 1 h 2 o for each element, we check if the number of atoms is balanced on both sides of the equation. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. Nahco3 = naoh +.
CO2 + NaOH Hấp thụ hoàn toàn 11,2 lít CO2 (đktc) vào dung dịch chứa
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one. Co 2 + naoh → na 2 co 3.
Na2CO3+H2O=NaOH+CO2 Balanced EquationSodium carbonate+Water=Sodium
Sodium bicarbonate = sodium hydroxide + carbon dioxide. Co 2 + naoh → na 2 co 3 + h 2 o (carbon dioxide) (sodium hydroxide) (sodium carbonate) (water) 2. (a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Nahco3 = naoh + co2 is a decomposition reaction where.
Co 2 + Naoh → Na 2 Co 3 + H 2 O (Carbon Dioxide) (Sodium Hydroxide) (Sodium Carbonate) (Water) 2.
(a) when the alkali ($\ce{naoh}$) solution is very dilute ($\mathrm{ph} < 8$), carbon dioxide will first react with water to form. Sodium bicarbonate = sodium hydroxide + carbon dioxide. Balancing of the reaction by hit and trial. Naoh + co2 = na2co3 + h2o is a double displacement (metathesis) reaction where two moles of sodium hydroxide [naoh] and one.
1 Naoh + 1 Co 2 = 1 Na 2 Co 3 + 1 H 2 O For Each Element, We Check If The Number Of Atoms Is Balanced On Both Sides Of The Equation.
Nahco3 = naoh + co2 is a decomposition reaction where one mole of.