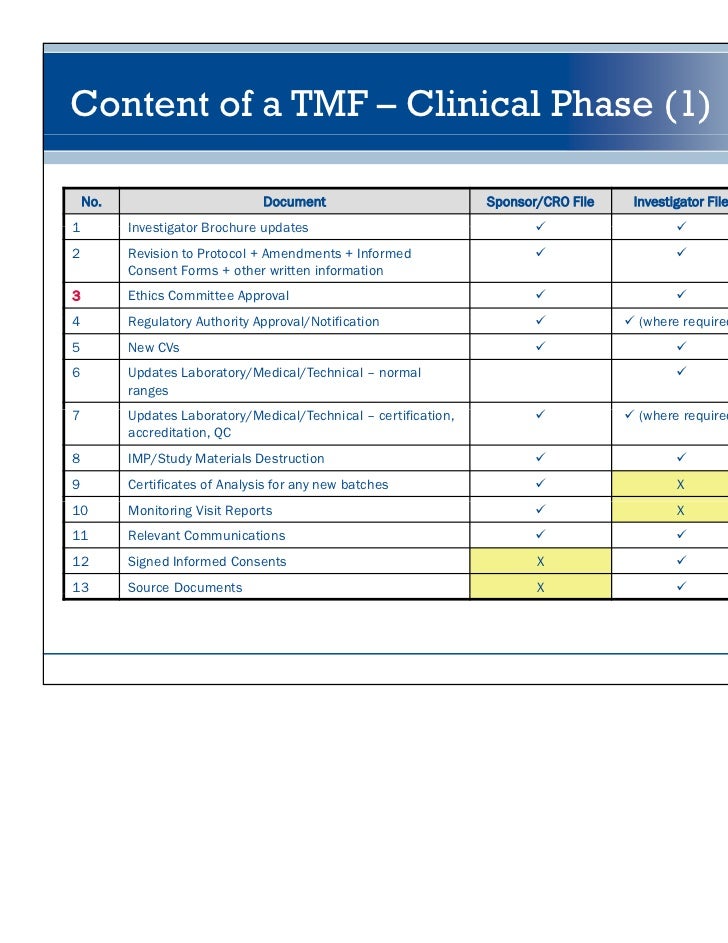
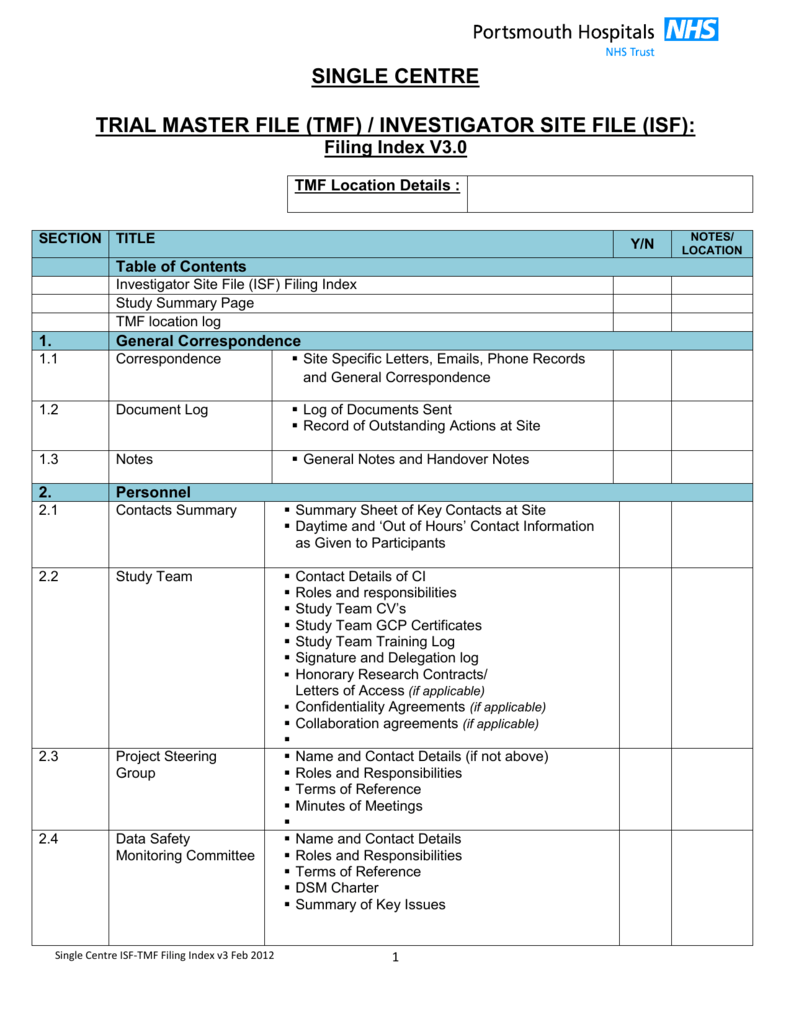
Trial Master File Template
Trial Master File Template - Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Please note that this page has been updated for 2015 following a quality check. Resources for current versions of the model. Trial master file (tmf) the complete set of documents as identified in the tmf index that individually and collectively permit evaluation. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library.
The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Resources for current versions of the model. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Trial master file (tmf) the complete set of documents as identified in the tmf index that individually and collectively permit evaluation. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library.
Resources for current versions of the model. Please note that this page has been updated for 2015 following a quality check. Trial master file (tmf) the complete set of documents as identified in the tmf index that individually and collectively permit evaluation. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data.
Trial Master File (TMF) Notion Template
Trial master file (tmf) the complete set of documents as identified in the tmf index that individually and collectively permit evaluation. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical.
Tmf Plan Template
Welcome to global health trials' tools and templates library. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Trial master file (tmf) the complete set of documents as identified in the tmf index that individually and collectively permit evaluation. Please note that this page has been.
Trial Master File / Investigator Site File Contents
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. The tmf plan template subgroup of the tmf reference model project is pleased to announce that.
CI Trial Master File Index
The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Welcome to global health trials' tools and templates library. Resources for current versions.
TABLE OF CONTENTS TRIAL MASTER FILE Single Profess Doc Template
The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Welcome to global health trials' tools and templates library. Resources for current versions of the model. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes.
Trial Master File PDF Audit Business
Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Resources for current versions of the model. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Welcome to global health trials' tools.
5 Pillars of the Trial Master File (TMF) LMK Clinical Research, LLC
Please note that this page has been updated for 2015 following a quality check. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the.
Trial Master File Checklist Template
Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. The tmf plan template subgroup of the tmf reference model project is pleased to.
Trial Master File TMF Clinical Trial Systems and FDA Expectations
The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Clinical trial protocol template this protocol template is designed to help research teams.
TrialMasterFile Checklist by Pharma Student Issuu
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. Resources for.
Resources For Current Versions Of The Model.
Essential documents are those documents that individually and collectively permit evaluation of the conduct of a trial and the quality of the data. The tmf plan template subgroup of the tmf reference model project is pleased to announce that the tmf plan template version 1.0 is now. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library.
Trial Master File (Tmf) The Complete Set Of Documents As Identified In The Tmf Index That Individually And Collectively Permit Evaluation.
Please note that this page has been updated for 2015 following a quality check.