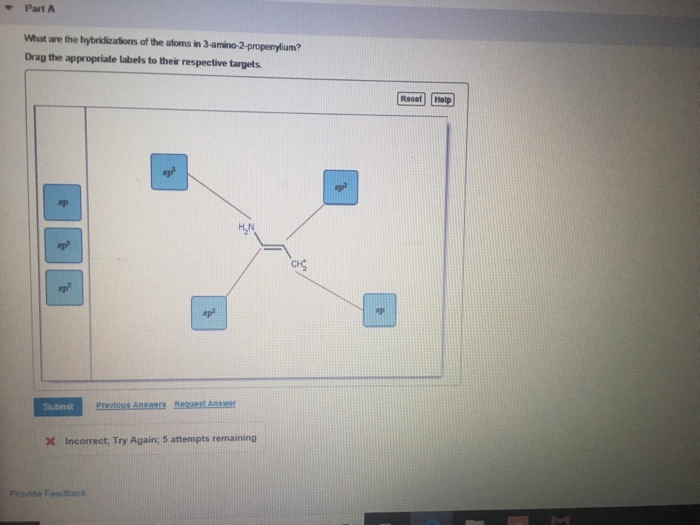
What Are The Hybridizations Of The Atoms In 3 Amino 2 Propenylium
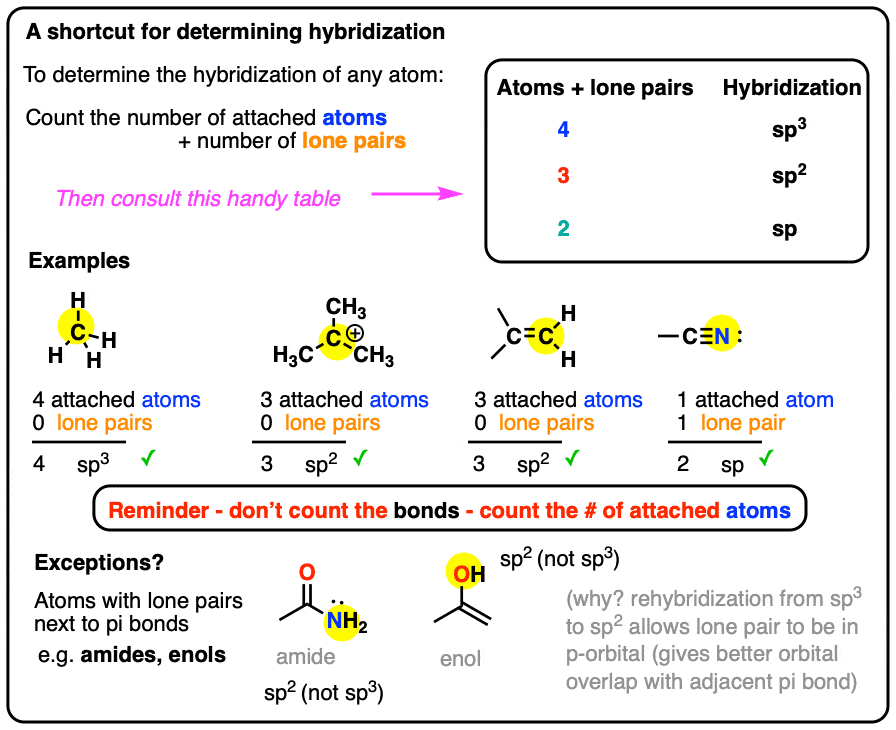
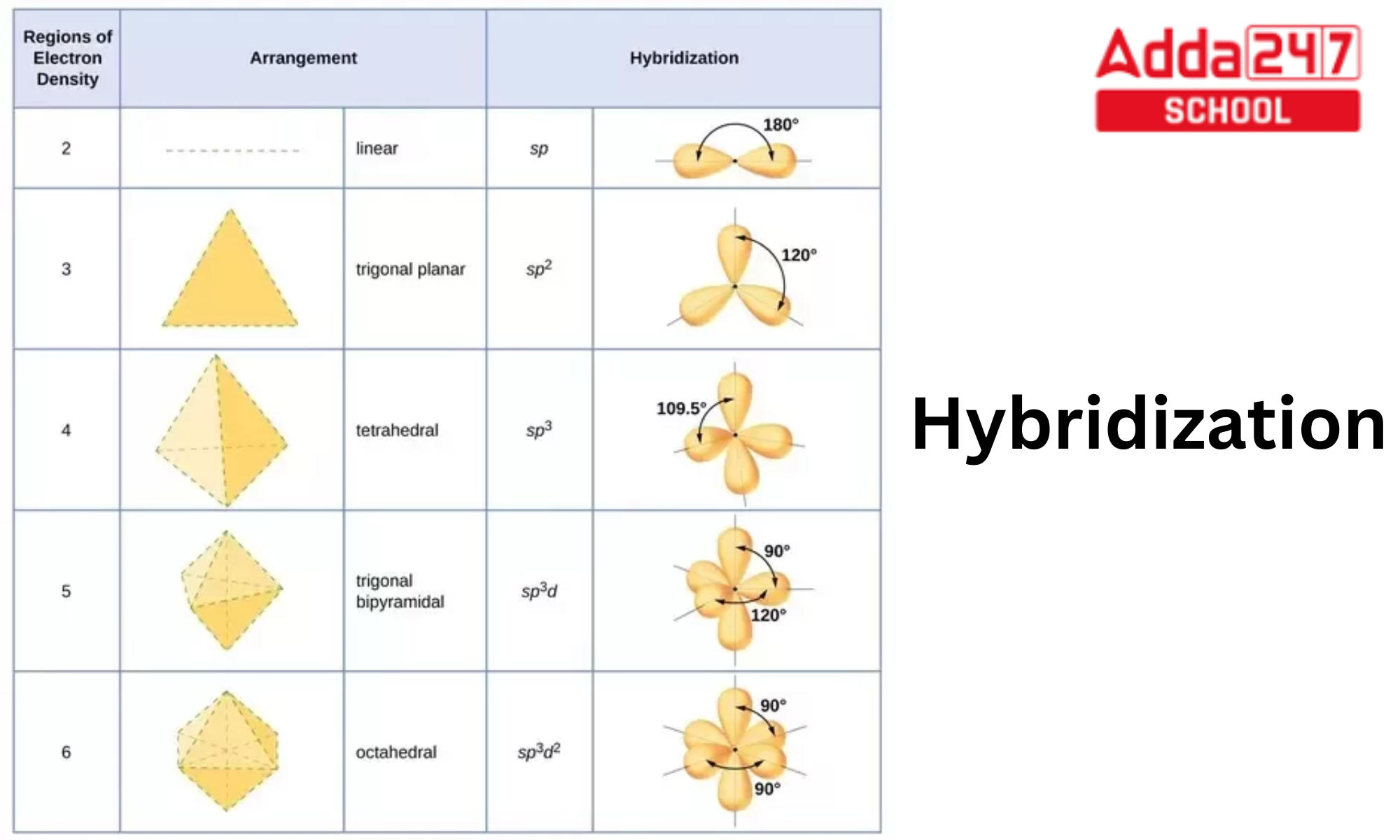
What Are The Hybridizations Of The Atoms In 3 Amino 2 Propenylium - If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. To put it plain, i can summarize the hybridizations in the following picture: 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. So, the 3 groups around the central atom gives you the sp 3. If there are 3 regions of electron density, the atom is sp2 hybridized. Drag the appropriate labels to. There are 2 steps to solve this one.
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drag the appropriate labels to. So, the 3 groups around the central atom gives you the sp 3. There are 2 steps to solve this one. If there are 2 regions of electron density, the atom is sp hybridized. If there are 3 regions of electron density, the atom is sp2 hybridized. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. To put it plain, i can summarize the hybridizations in the following picture:
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. So, the 3 groups around the central atom gives you the sp 3. There are 2 steps to solve this one. To put it plain, i can summarize the hybridizations in the following picture: 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. Drag the appropriate labels to. If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized.
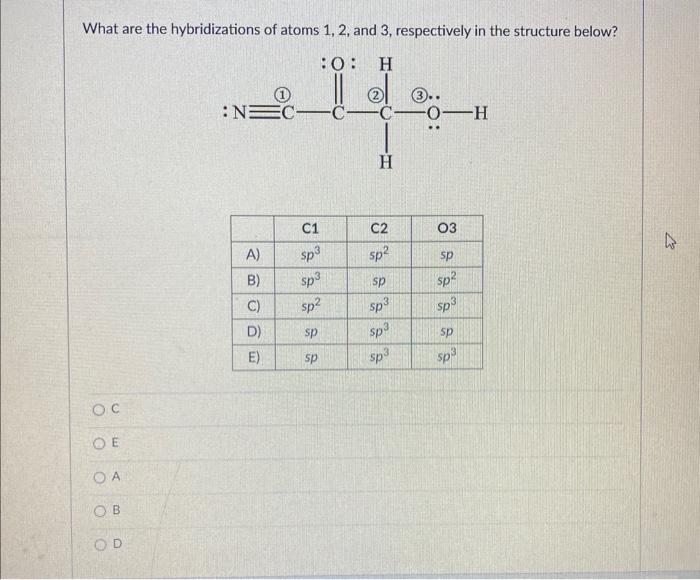
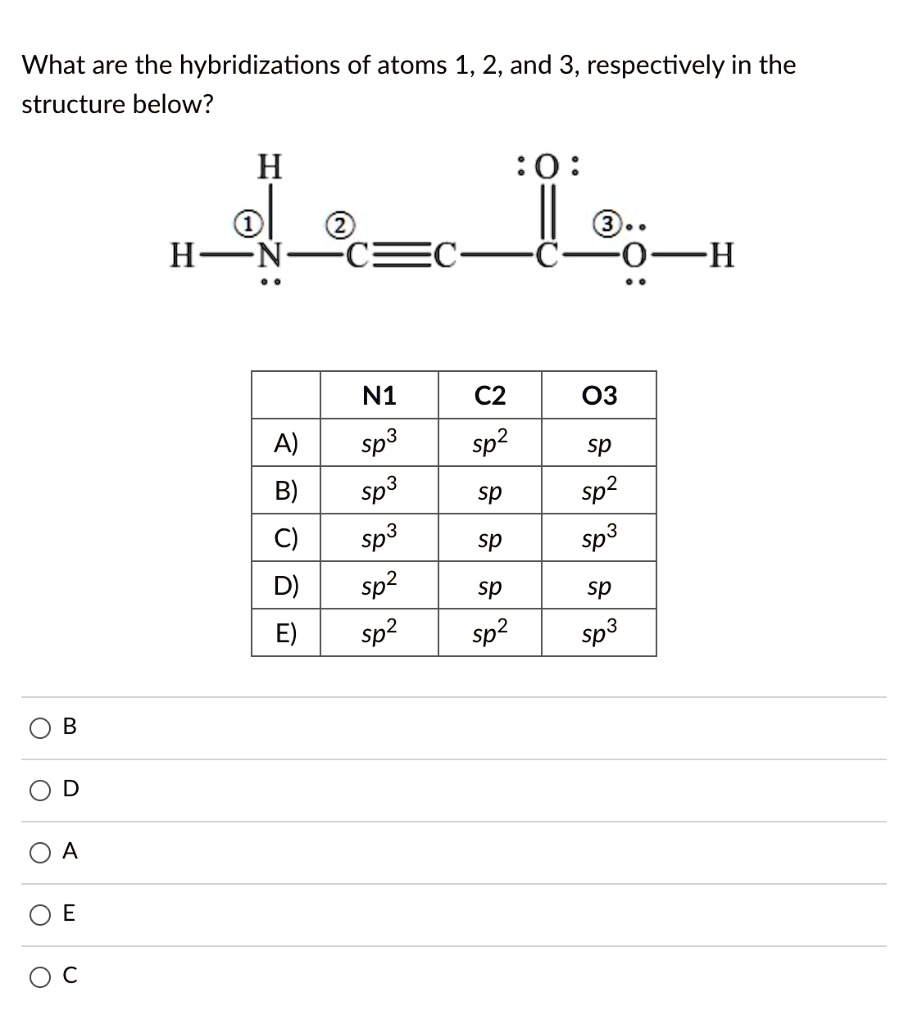
Solved What are the hybridizations of atoms 1,2 , and 3 ,
8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. To put it plain, i can summarize the hybridizations in the following picture: There are 2 steps to solve this one. If there are 3 regions of electron density, the atom is sp2 hybridized. If there.
میانبر استاد شیمی آلی تولیدی فرمیک
Drag the appropriate labels to. If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If there are 3 regions of electron density, the atom is sp2 hybridized. So, the 3 groups around the central atom gives you the sp 3.
Solved What are the hybridizations of atoms 1,2 , and 3 ,
If there are 3 regions of electron density, the atom is sp2 hybridized. There are 2 steps to solve this one. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. Drag the appropriate labels to. So, the 3 groups around the central atom gives you.
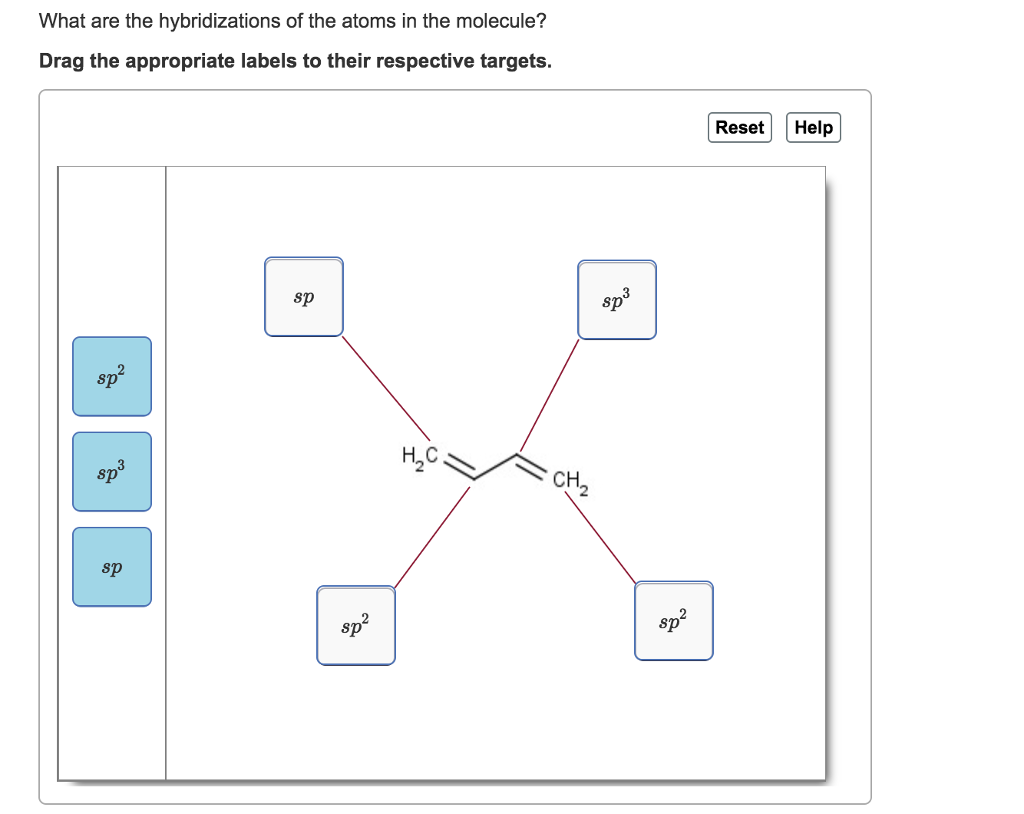
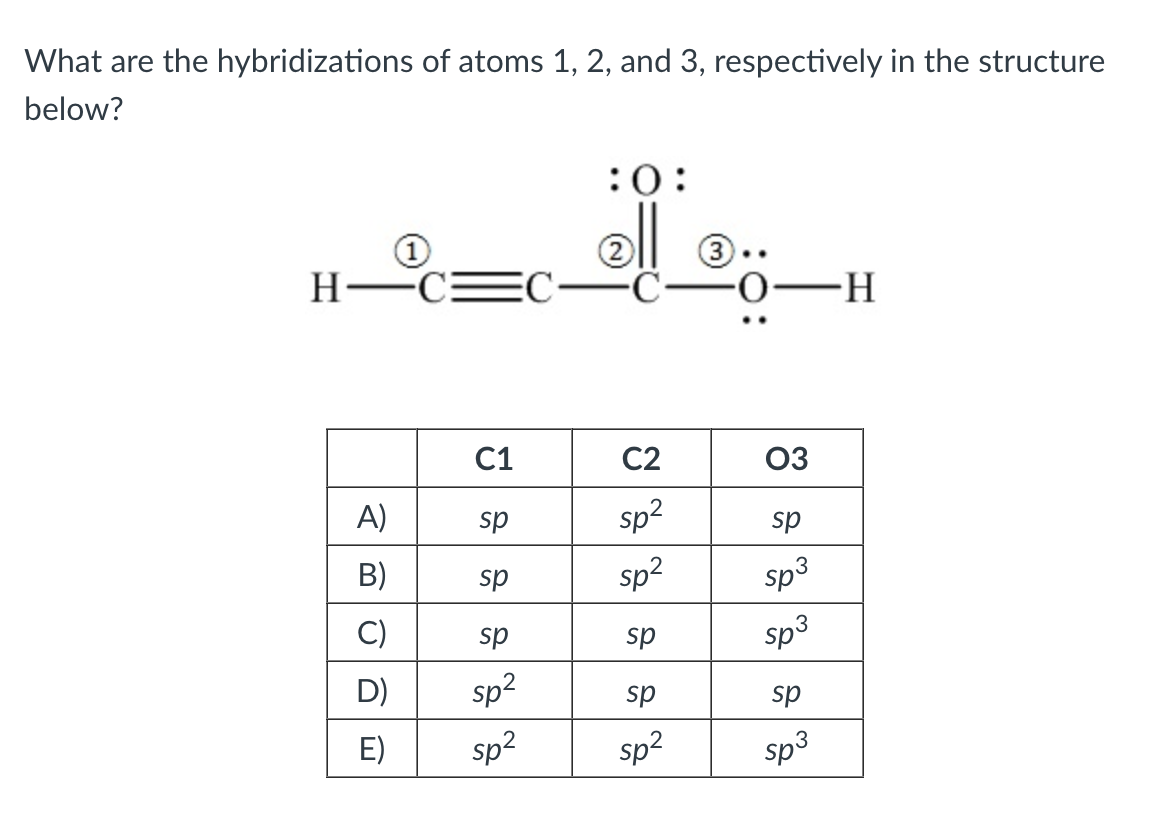
Solved What are the hybridizations of the atoms in the
To put it plain, i can summarize the hybridizations in the following picture: If there are 2 regions of electron density, the atom is sp hybridized. If there are 3 regions of electron density, the atom is sp2 hybridized. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms.
What is Hybridization? sp3, sp2, Examples and Formula
So, the 3 groups around the central atom gives you the sp 3. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. If there are 2 regions of electron density, the atom is sp hybridized. If there are 3 regions of electron density, the atom.
Hybridization Orbitals Chart
If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Drag the appropriate labels to. So, the 3 groups around the central atom gives you the sp 3.
Types of Hybridization Definitions, Examples, Key Features, Steps to
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized. There are 2 steps to solve this one. So, the 3 groups around the central atom gives you.
what are the hybridizations of atoms 1 2and 3 respectively in the
Drag the appropriate labels to. To put it plain, i can summarize the hybridizations in the following picture: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. If there are 2 regions of electron density, the atom is sp hybridized. 8 rows the shapes of organic molecules may be understood by looking at.
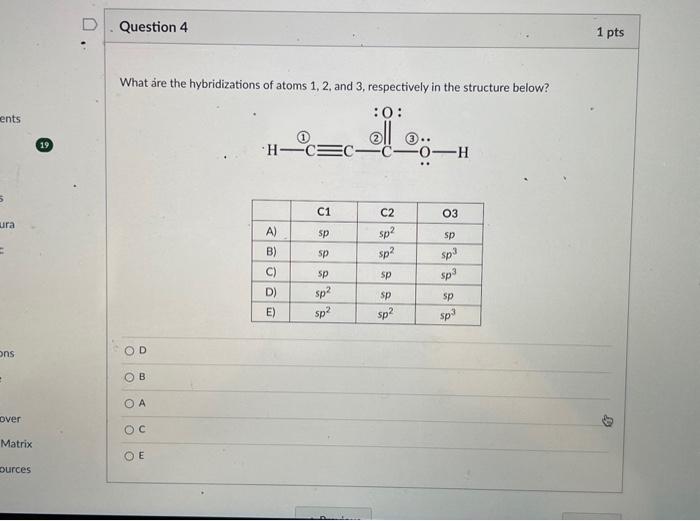
Solved What are the hybridizations of atoms 1,2 , and 3 ,
If there are 2 regions of electron density, the atom is sp hybridized. Drag the appropriate labels to. There are 2 steps to solve this one. If there are 3 regions of electron density, the atom is sp2 hybridized. To put it plain, i can summarize the hybridizations in the following picture:
Solved I posted this question once before and they got it
So, the 3 groups around the central atom gives you the sp 3. 8 rows the shapes of organic molecules may be understood by looking at the hybridization adopted by each of the atoms in the molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. There are 2 steps to solve this.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
If there are 3 regions of electron density, the atom is sp2 hybridized. If there are 2 regions of electron density, the atom is sp hybridized. Drag the appropriate labels to. To put it plain, i can summarize the hybridizations in the following picture:
8 Rows The Shapes Of Organic Molecules May Be Understood By Looking At The Hybridization Adopted By Each Of The Atoms In The Molecule.
So, the 3 groups around the central atom gives you the sp 3. There are 2 steps to solve this one.