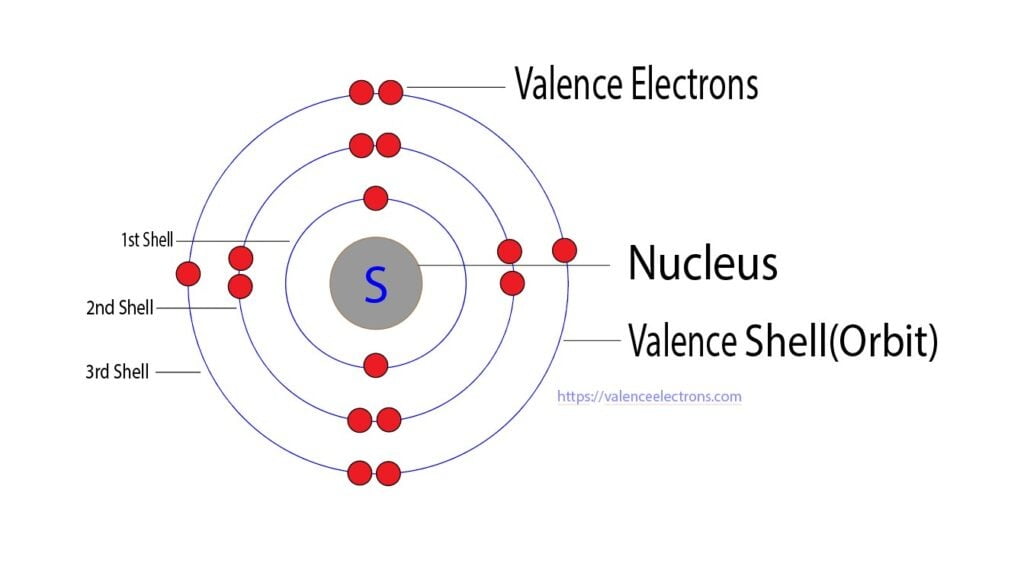
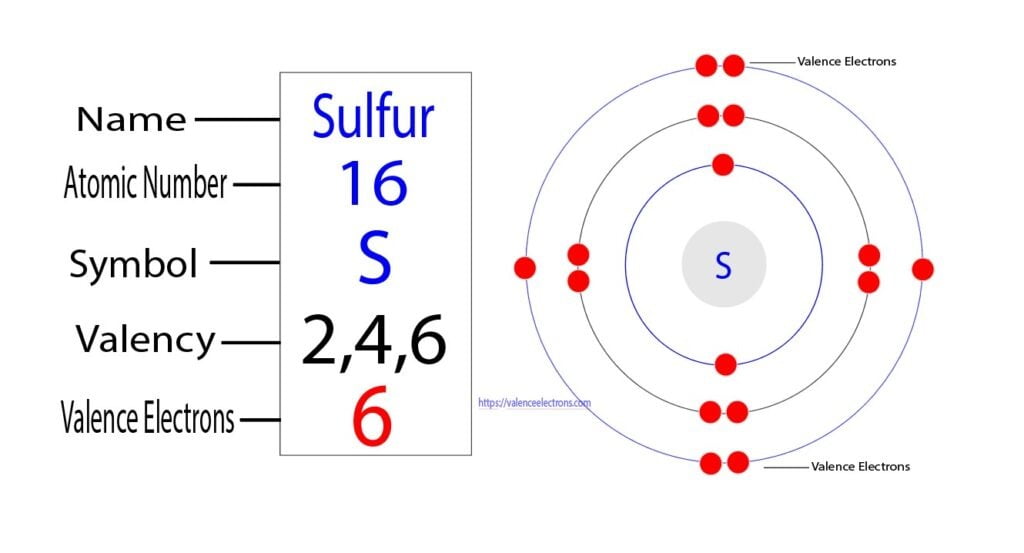
What Charge Does Sulfur Have
What Charge Does Sulfur Have - This is because it needs to gain two electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have? When a sulfur (s) atom becomes an ion, what charge does it usually have?. Sulfide ions will have two more. Chemical elements have the electrons in their orbitals. What is the charge of sulfur? For a stable outer shell, it needs to acquire two more electrons. The electronic configuration of sulfur is 2, 8, 6.
For a stable outer shell, it needs to acquire two more electrons. This is because it needs to gain two electrons. What is the charge of sulfur? The electronic configuration of sulfur is 2, 8, 6. When a sulfur (s) atom becomes an ion, what charge does it usually have? Sulfide ions will have two more. Chemical elements have the electrons in their orbitals. When a sulfur (s) atom becomes an ion, what charge does it usually have?.
What is the charge of sulfur? When a sulfur (s) atom becomes an ion, what charge does it usually have?. When a sulfur (s) atom becomes an ion, what charge does it usually have? The electronic configuration of sulfur is 2, 8, 6. For a stable outer shell, it needs to acquire two more electrons. This is because it needs to gain two electrons. Sulfide ions will have two more. Chemical elements have the electrons in their orbitals.
Diagram representation of the element sulfur Vector Image
The electronic configuration of sulfur is 2, 8, 6. For a stable outer shell, it needs to acquire two more electrons. Sulfide ions will have two more. When a sulfur (s) atom becomes an ion, what charge does it usually have? What is the charge of sulfur?
Does sulfur have 8 electrons? YouTube
When a sulfur (s) atom becomes an ion, what charge does it usually have? When a sulfur (s) atom becomes an ion, what charge does it usually have?. The electronic configuration of sulfur is 2, 8, 6. Chemical elements have the electrons in their orbitals. Sulfide ions will have two more.
How Many Valence Electrons Does Sulfur (S) Have?
What is the charge of sulfur? The electronic configuration of sulfur is 2, 8, 6. When a sulfur (s) atom becomes an ion, what charge does it usually have? For a stable outer shell, it needs to acquire two more electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have?.
How Many Valence Electrons Does Sulfur Hexafluoride Have?
When a sulfur (s) atom becomes an ion, what charge does it usually have? The electronic configuration of sulfur is 2, 8, 6. What is the charge of sulfur? When a sulfur (s) atom becomes an ion, what charge does it usually have?. This is because it needs to gain two electrons.
I number of electrons howlasopa
When a sulfur (s) atom becomes an ion, what charge does it usually have? The electronic configuration of sulfur is 2, 8, 6. Chemical elements have the electrons in their orbitals. Sulfide ions will have two more. For a stable outer shell, it needs to acquire two more electrons.
How does sulfur have 6 valence electrons? YouTube
When a sulfur (s) atom becomes an ion, what charge does it usually have?. For a stable outer shell, it needs to acquire two more electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have? The electronic configuration of sulfur is 2, 8, 6. What is the charge of sulfur?
Sulfur, atomic structure Stock Image C018/3697 Science Photo
For a stable outer shell, it needs to acquire two more electrons. Chemical elements have the electrons in their orbitals. The electronic configuration of sulfur is 2, 8, 6. This is because it needs to gain two electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have?.
Periodic Table Sulfur Charge Periodic Table Timeline
For a stable outer shell, it needs to acquire two more electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have?. Chemical elements have the electrons in their orbitals. Sulfide ions will have two more. This is because it needs to gain two electrons.
Why does sulfur have 12 valence electrons? YouTube
When a sulfur (s) atom becomes an ion, what charge does it usually have?. Sulfide ions will have two more. This is because it needs to gain two electrons. What is the charge of sulfur? When a sulfur (s) atom becomes an ion, what charge does it usually have?
sulfur Definition, Element, Symbol, Uses, & Facts Britannica
For a stable outer shell, it needs to acquire two more electrons. When a sulfur (s) atom becomes an ion, what charge does it usually have? The electronic configuration of sulfur is 2, 8, 6. This is because it needs to gain two electrons. What is the charge of sulfur?
What Is The Charge Of Sulfur?
Chemical elements have the electrons in their orbitals. This is because it needs to gain two electrons. Sulfide ions will have two more. When a sulfur (s) atom becomes an ion, what charge does it usually have?
When A Sulfur (S) Atom Becomes An Ion, What Charge Does It Usually Have?.
The electronic configuration of sulfur is 2, 8, 6. For a stable outer shell, it needs to acquire two more electrons.