What Two Substances Form From An Acid Base Neutralization
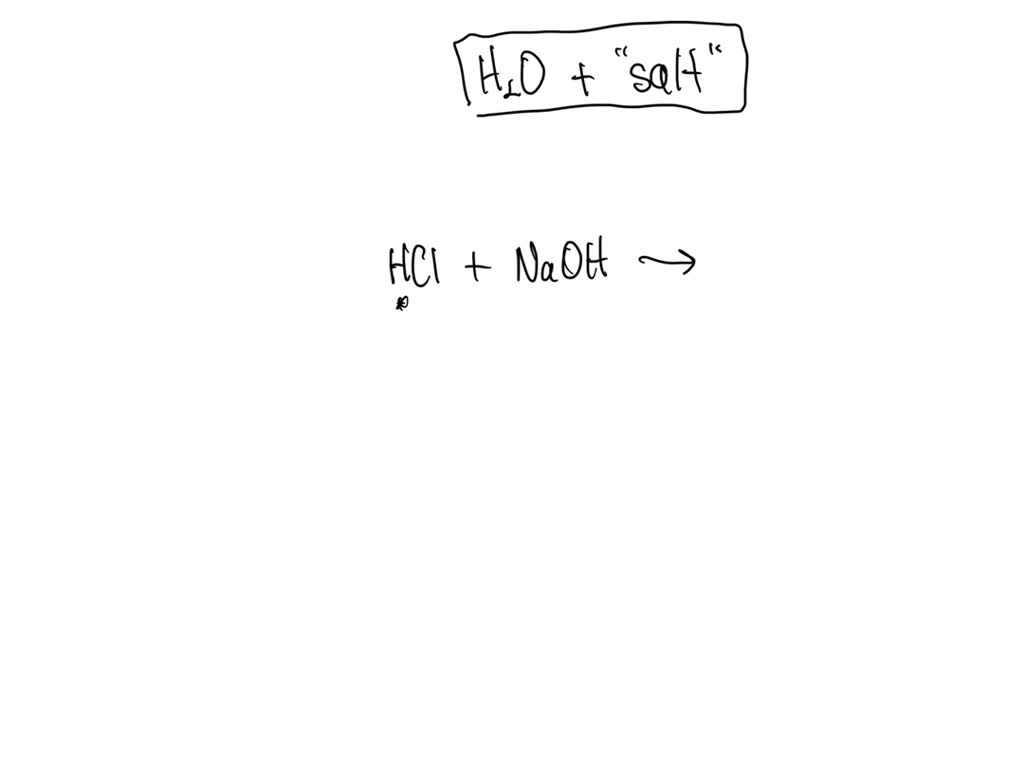
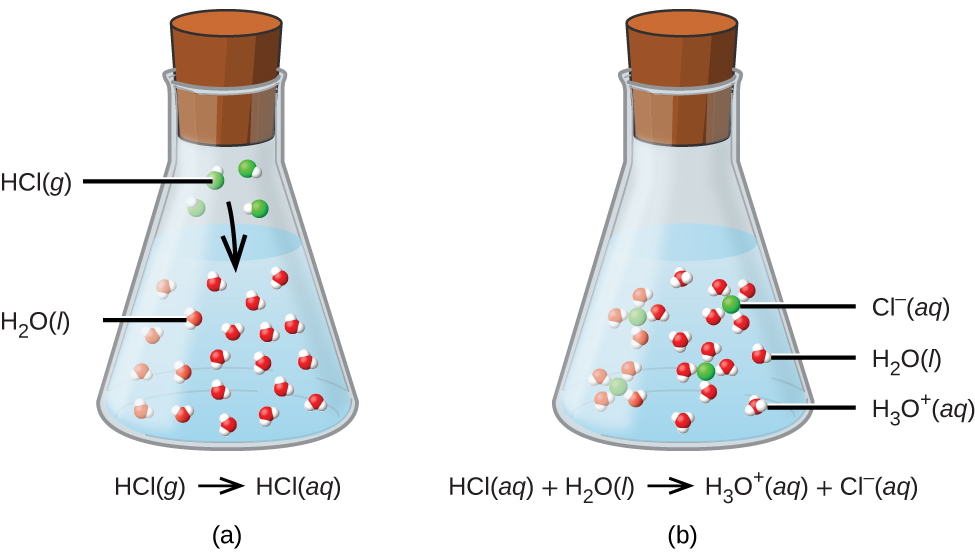
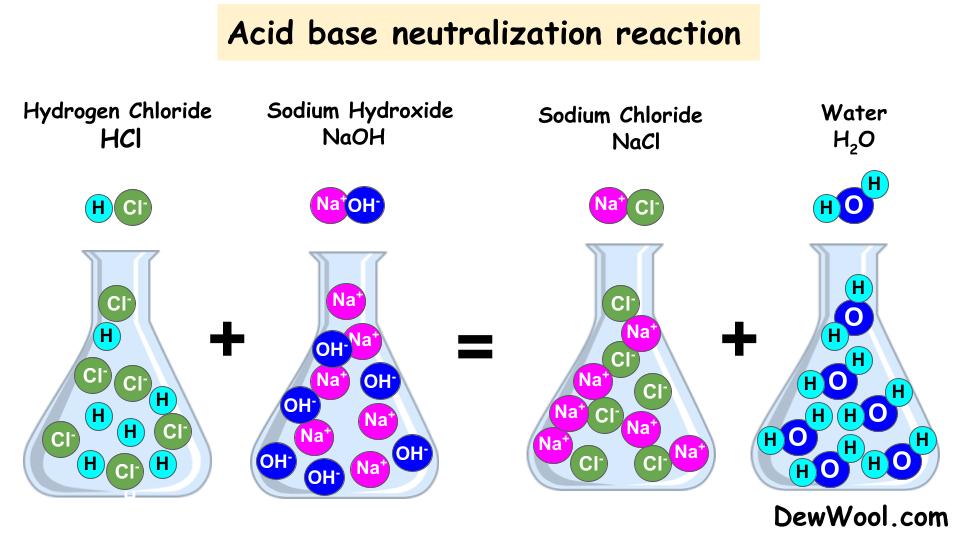
What Two Substances Form From An Acid Base Neutralization - They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have another property:
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property:
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity.
Acid Base Neutralization Reactions Chemistry YouTube
Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called.
SOLVED Which substances are always produced in an acidbase
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. Acids and bases have another property: In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called.
Acid/Base Neutralization Reactions & Net Ionic Equations YouTube
They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and.
Neutralization Reaction Diagram
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
Concepts of Acid Base Neutralization PDF Acid Ph
They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and.
Neutralization Reaction Definition, Equation and Examples Teachoo
Acids and bases have another property: They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and.
Type Of Acid Base Neutralization Reactions
Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. They react with each other to make water and an ionic compound called a salt. In general, a neutralization reaction is a type of double replacement reaction between an acid and.
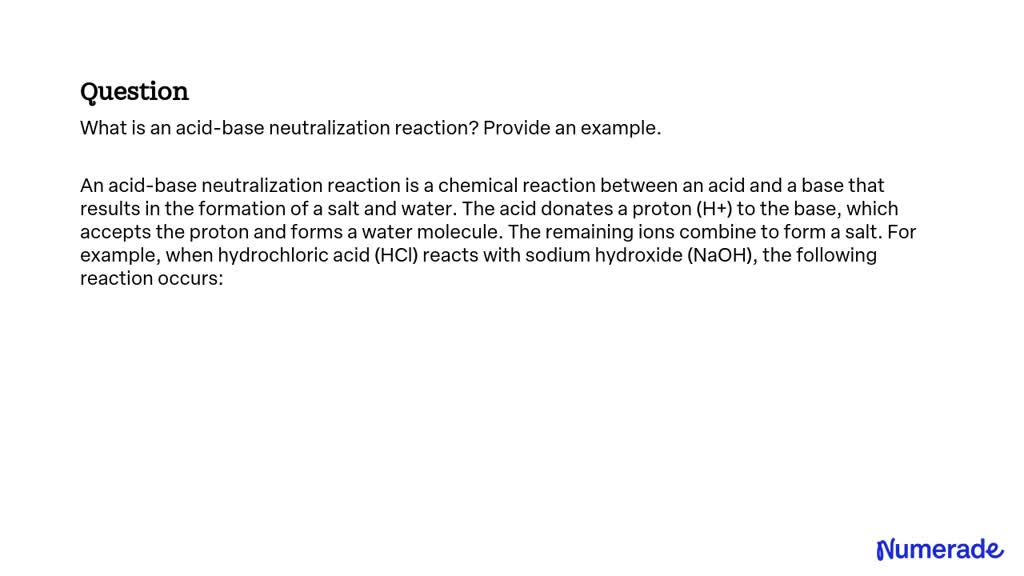
SOLVED What is an acidbase neutralization reaction? Provide an example.
Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called.
Acidbase neutralization reaction, illustration Stock Image F027
In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. They react with each other to make water and an ionic compound called a salt. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. Acids and bases have.
What is Neutralisation in Chemistry? The Chemistry Blog
In general, a neutralization reaction is a type of double replacement reaction between an acid and a base. They react with each other to make water and an ionic compound called a salt. Acids and bases have another property: In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an.
Acids And Bases Have Another Property:
They react with each other to make water and an ionic compound called a salt. In chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity. In general, a neutralization reaction is a type of double replacement reaction between an acid and a base.