Which Combination Of Atoms Can Form A Polar Covalent Bond
Which Combination Of Atoms Can Form A Polar Covalent Bond - Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. This occurs when one atom has a higher. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond.
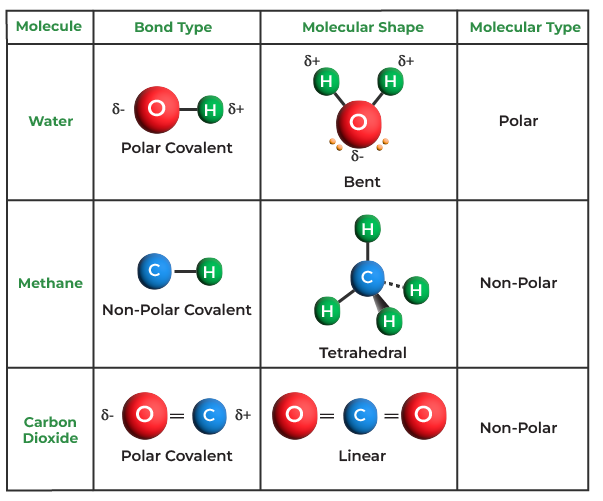
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. A polar covalent bond is created when the shared electrons between atoms are not equally shared. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. This occurs when one atom has a higher. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms.
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Only h and br form polar covalent bond. This occurs when one atom has a higher. Covalent bonding occurs when pairs of electrons are shared by atoms. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms are not equally shared.
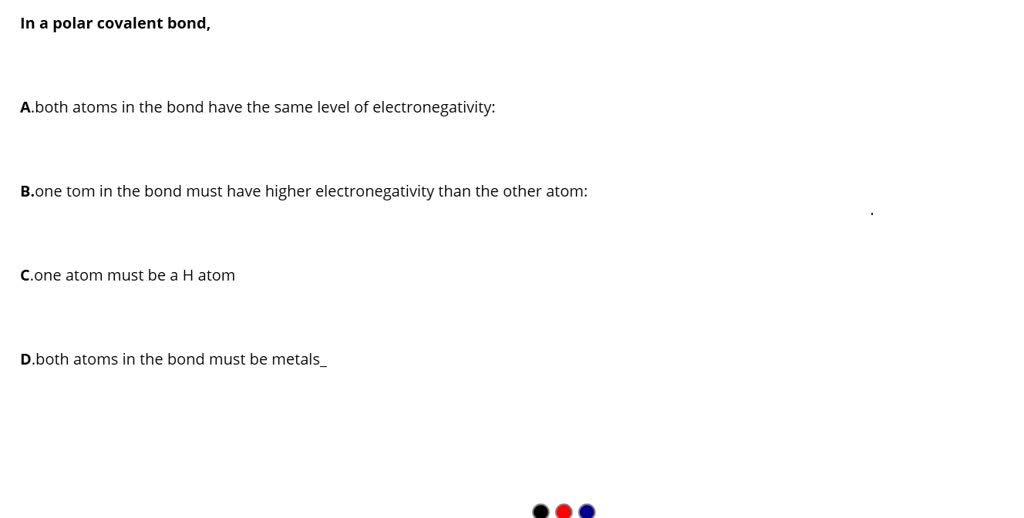
SOLVED In a polar covalent bond, A) both atoms in the bond have the
एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. This occurs when one atom has a higher. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms.
Polar Covalent Bonds Clearly Explained for Easy Learning
Only h and br form polar covalent bond. This occurs when one atom has a higher. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Explain why the covalent bonds between , oxygen and hydrogen atoms in h.
Reading Covalent Bonds Biology I
As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. Only h and br form polar covalent bond. Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. A polar covalent bond is created when the shared electrons between atoms are.
Solved Question 1 (1 point)A polar covalent bond would form
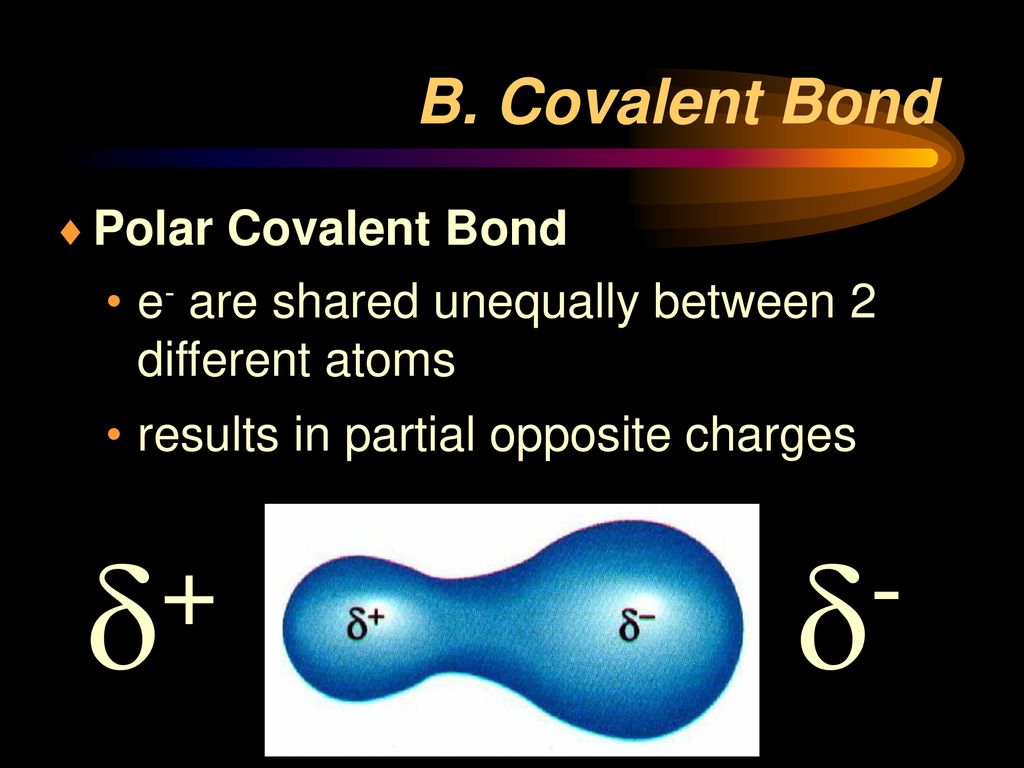
A polar covalent bond is created when the shared electrons between atoms are not equally shared. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. This occurs when one atom has a higher. Only h and br form polar covalent bond.
Polar Covalent Bond Definition And Examples, 49 OFF
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. Only h and br form polar covalent bond. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms are not equally.
Definition and Examples of a Polar Bond
Covalent bonding occurs when pairs of electrons are shared by atoms. Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms.
Covalent Bond Definition and Examples
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between atoms, within a. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Covalent bonding occurs when pairs of electrons.
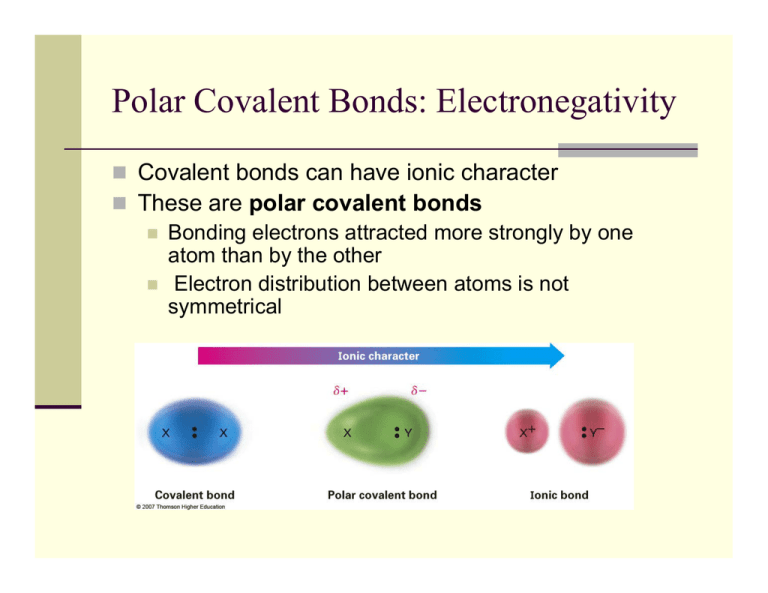
Polar Covalent Bonds Electronegativity
Only h and br form polar covalent bond. A polar covalent bond is created when the shared electrons between atoms are not equally shared. Covalent bonding occurs when pairs of electrons are shared by atoms. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons between.
II. Kinds of Chemical Bonds Ionic Bond Covalent Bond Comparison Chart
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. Covalent bonding occurs when pairs of electrons are shared by atoms. A polar covalent bond is created when the shared electrons between atoms are not equally shared. As demonstrated below, bond polarity is a useful concept for describing the sharing of electrons.
As Demonstrated Below, Bond Polarity Is A Useful Concept For Describing The Sharing Of Electrons Between Atoms, Within A.
Explain why the covalent bonds between , oxygen and hydrogen atoms in h 2 o molecule are polar. एक कार्ब अणू __________ सहसंयुज बंध तयार करू शकतो. This occurs when one atom has a higher. A polar covalent bond is created when the shared electrons between atoms are not equally shared.
Only H And Br Form Polar Covalent Bond.
Covalent bonding occurs when pairs of electrons are shared by atoms.




/PolarConvalentBond-58a715be3df78c345b77b57d.jpg)