Why Do Metals Form Cations
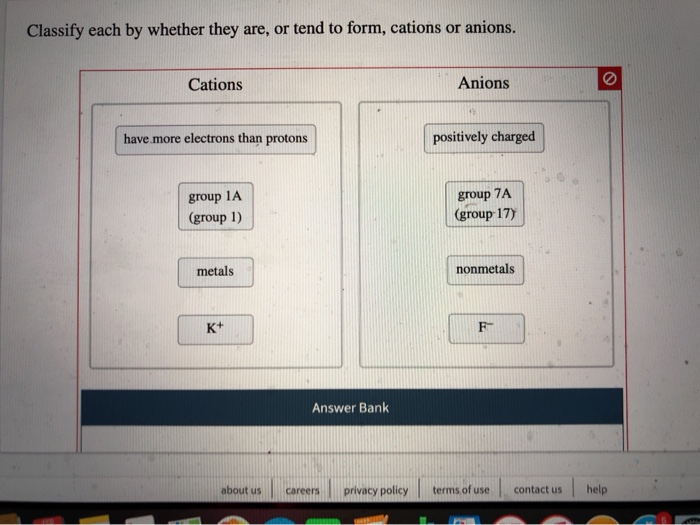
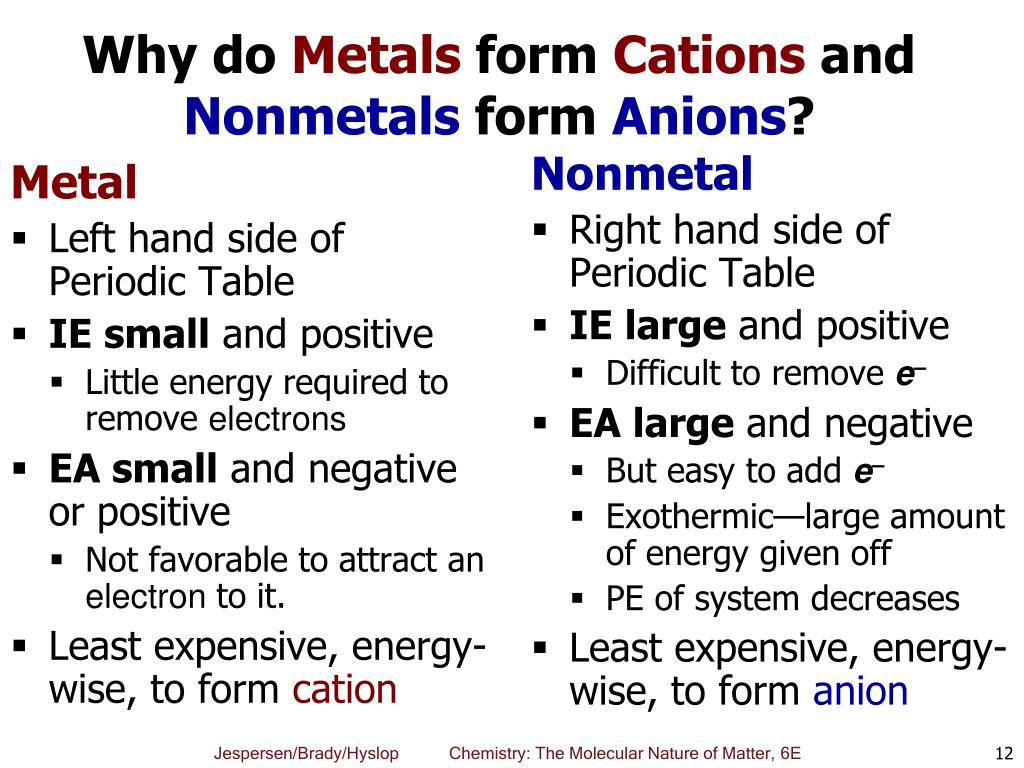
Why Do Metals Form Cations - Cations are formed when a neutral atom loses an electron. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal.
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. In more detail, the formation of. Cations are formed when a neutral atom loses an electron. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge.
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Metals are prone to losing electrons as a result of the arrangement of. Cations are formed when a neutral atom loses an electron. In more detail, the formation of.
Metals Tend to Form Cations or Anions IndiahasPeck
Cations are formed when a neutral atom loses an electron. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms.
Solved Question 6 (1 point) Why do metals tend to form
Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. In more detail, the formation of. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend.
Do Metals Form Anions Or Cations
Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Metals are prone to losing electrons as a.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Cations are formed when a.
Ionic Compounds Stone Cold Chemistry Talk
In more detail, the formation of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Cations are formed when a neutral atom loses an electron. Metals are.
Cations and Anions Definitions, Examples, and Differences
In more detail, the formation of. Cations are formed when a neutral atom loses an electron. Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one.
13 Transition Metals & Colored Complexes The!Mad!Scientist!
Cations are formed when a neutral atom loses an electron. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a.
Which Metals Form Cations With Varying Positive Charges TerryhasYoder
Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. Metals are prone to losing electrons as a result of the arrangement of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Cations are formed when a.
What are the properties of most metals? ppt download
In more detail, the formation of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a result of the arrangement.
Explain why almost all metals tend to form cations. Numerade
Metals are prone to losing electrons as a result of the arrangement of. Metals form positive ions because they tend to lose electrons during chemical reactions, resulting in a positive charge. In more detail, the formation of. Cations are formed when a neutral atom loses an electron. First, each element that forms a cation is a metal, except for one.
Metals Form Positive Ions Because They Tend To Lose Electrons During Chemical Reactions, Resulting In A Positive Charge.
Cations are formed when a neutral atom loses an electron. In more detail, the formation of. First, each element that forms a cation is a metal, except for one (hydrogen), while each element that forms an anion is a nonmetal. Metals are prone to losing electrons as a result of the arrangement of.




.jpg)